|
|
Post by evan cornish-keefe on Sept 20, 2015 21:51:50 GMT -6
Hi Tom, Sorry for the delayed response....
I'd be interested to see what information you're reading about temperatures at which materials like lithium are most active fluxes.
My understanding is that Lithium, Sodium, and Potassium are all metals.
I have over 16% Lithium Carb. in that glaze, I've had Titanite crystals form in a cone 6 glaze without Lithium, but the RO had to be equally as high (using sodium instead of lithium) to keep the number of crystals down. I've been firing to 2216f, often closer to cone 5, this glaze is extremely fluid for sure.
I don't think I've seen the white/grey spots you mentioned?
Have you timed how long it takes your kilns to cool naturally from peak temp?
|
|
|
|
Post by tileman2 on Sept 21, 2015 21:02:29 GMT -6
Evan:
Like so many chemicals/minerals we use: sodium and potassium can be confusing. They are classified as alkali metals; but they do not occur in nature as metals, but rather they have to be compounded to produce metals. Even then the metal is so soft you can cut it with a knife, and it has to be stored in oil because exposure to air will oxidize it rapidly.( like lithium) They occur in nature as salts; in our applications they are in fact mineral salts such as feldspar; and they are also compounded into potassium, sodium, and lithium hydroxides and carbonates. So I guess the answer is yes they are metals, and yes they are salts: but in our application a vigorous mineral / salt with a metallic anion. I keep all the sodium and aluminum I can out of my recipe and clay.
Will have to go back through my files upstairs and find the papers from a professor at the University of Illinois @ Campaign-Urbana. He works more with industrial ceramics, but has written extensively about fluxes including lithium.
In regards to kilns: my 6.5 Cf top loader takes almost 16 hours to drop from 1865F to 220F or so. It takes almost 30 minutes to drop from 2230F to 1950F- I have to pull the top bunge to get it to drop that fast. I do however use ceramic/fiber rope around the top to seal the lid. My 1.75CF test / small piece kiln will drop from 1865F to 220F in less than 10 hours. My 16CF super dragon I use primarily to bisque and do standard cone 6; it will drop from 1865F to 200F in 8-10 hours; but I have a vent on it to help it along. When the kilns hit 800-900F, I use small shims to create a 1/8 to 1/4 gap to make them cool a bit faster without allowing too much cool air in.
Going to run a few reduction experiments in October, but for the next month or so all I will be doing is making custom tile setters. I need four different sizes; and somewhere between 40-50 of each size. I drew out the interior chamber sizes of my three primary firing kilns; and designed custom setters that maximize the space in each. They are individual setters than can be stacked; but also designed to allow air to flow around, under, and through them to help prevent help hot and cold spots. Modified a good high alumina clay recipe that has very little expansion, and is light weight.
Tom
|
|
|
|
Post by evan cornish-keefe on Sept 25, 2015 23:45:16 GMT -6
Yeah, that sounds more accurate. My understanding of chemistry is only elementary, so I'm not sure how it being a salt or not is relevant.
What I think is important is how similar&different lithium, sodium and potassium are acting in the glaze.
My last couple firings are leading me to believe that something about the clay body is the most significant factor causing these rough crystals. I've been messing with a variety of commercial clays,all advertised as being for the cone 6 range, though it seems to me that some should be fired under cone 6 and others as more like cone 8 or 10 or ?......
|
|
|
|
Post by tileman2 on Sept 26, 2015 14:00:57 GMT -6
Evan:
The big issue I have seen as of late is changes in material / chemical supplies. A national supplier decided to dump most of its traditional suppliers and mines and decided to buy from China. Apparently that extra ten cents a pound was worth it to them. That occurred several months back, and I have been hearing and seeing a continuous list of complaints since it occurred. Continuity of ingredients I suspect will be a problem for many months to come. The only real difference I have seen between cone 6 and cone 10 clay is the amount of feldspar (flux) used: speaking more so about porcelain than others. Been playing around with my own clay mixes for some time now: works great for flat work but worthless for throwing.
Tom
|
|
|
|
Post by tileman2 on Sept 29, 2015 16:39:49 GMT -6
Evan:
Do you by chance have pics of standard cone 6 zinc/silica glaze that has 9 plus percent of lithium included? I have tested up to 8; the changes being fluidity, but more so causing the zinc to boil off. I recently bumped to 4%, but noticed copper and cobalt were showing signs of boil off at 2230F. [1270C] I fired again at 2222F, which helped with the boil off: but did not like the way the colorants blended. I know I can fire lower, but prefer to fire higher because it seems to allow the colorants to blend better. Maybe I will just go back to 3% and 2232F.
Tom
|
|
|
|
Post by evan cornish-keefe on Sept 29, 2015 23:31:18 GMT -6
The highest % lithium i have tested in a zinc silicate crystal glaze (with a typical crystal & glass ratio) is 9.46%, all i have is a little test tile. I don't plan to use this glaze again because personally I prefer having some alumina in the recipe. I look at glazes more in terms of the UMF and don't think percentage indicates as much, but i often use as much as 25% EPK in an otherwise similar recipe. Lots of my pieces have bloating and pinholes with clay bodies that are over-fired at cone 6, but I don't think i've seen boil off, do you have any pictures of it? I've been slowly lowering my peak temp, mostly in response to switching clay bodies, started at 2232 and am now at 2190 - cone 5 1/2 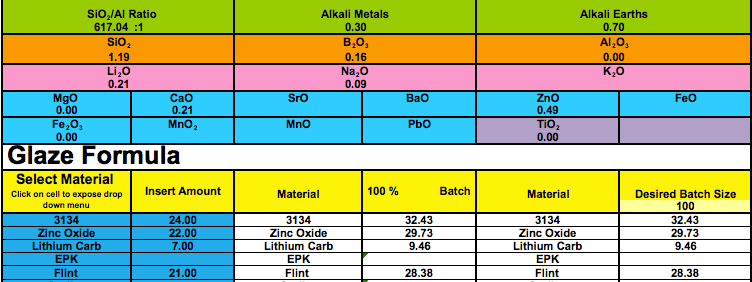 |
|
|
|
Post by tileman2 on Sept 30, 2015 20:53:12 GMT -6
Evan:
Will snap a few pics this weekend and get them posted.
Tom
|
|
|
|
Post by tileman2 on Oct 3, 2015 21:26:13 GMT -6
Evan: These are the examples of "boil off"- which is actually zinc evaporation. French process zinc is made from zinc vapors, so it is much more susceptible to boil off than zinc processed from sphalerite (yellow zinc.) The first time I fired my new french process zinc was to 2250F- with this radical result. (extreme boil off)  Then I lowered temp to 2230F: and I get minor patches only with CO and CU.  Very small area to the left of the camera flash. I run most colorants to 2230F because it makes the glaze more fluid and mixes better. Recently I have been lowering Cu and CO down to 2225F to keep these small patches off. Edit note: both examples have 4% lithium additions. No alumina, no additional fluxes. Tom |
|
|
|
Post by evan cornish-keefe on Oct 5, 2015 21:57:12 GMT -6
I'm not familiar with the different types of zinc?
From the photos it doesn't look like the zinc is boiling, would you share the clay and glaze recipe?
|
|
|
|
Post by tileman2 on Oct 6, 2015 17:43:19 GMT -6
Evan:
The clay is cone 6 porcelain with 10% molochite and 5% #6 tile kaolin. I do mostly flat work, so I do not want the plasticity.
Glaze: Frit 3110 54%
French zinc 27%
SiO2 20%
EPK/A10 5%
Lith. Carb 4%
Very simple recipe- the ZNO and SiO2 are both high purity: 99.8% - not purchased through the ceramic industry. I have found that when you fire to cone 10; the purity levels are not required because the additional heat burns off/runs off alot of it. However, when you do flat work at cone 6 the impurities effect the glaze. I also use Bentolite A-10 because it is higher purity, much less sodium and alumina. I have seen the same type of boil off when I use metallic zinc- which is the purest form of zinc. I do not make any TiO2 additions as well: the purity levels make the recipe highly reactive to TiO2. After I mix the glaze, I put less than a pinhead 0.05% of TiO2.
Tom
|
|
|
|
Post by tileman2 on Nov 16, 2015 19:55:09 GMT -6
Recipe Tweaking- Tile 1 = 25% ZNO Tile 2: 26% ZNO Tile 3 (left hand) 27% ZNO  Notes: While reducing ZNO to control crystal population works well: too much reduction also effects crystal size. The second picture has nothing to do with testing: just happen to like the color that Neodymium Oxide produces- just adding a pinch of praseodymium oxide really enriches the color. Holly: a pinch is equal to two whispers.  Tom |
|
|
|
Post by tileman2 on Nov 25, 2015 17:49:04 GMT -6
Think I am going to call this Celestial, or Timothy Leary is not dead. Looks rough, looks textured, looks really weird: but it is smooth, glossy, and still weird.  Yttrium. CoCarb, and Vandium Edit: The dark blues, medium blue patches are not shadows: they are in the tile. The white patches are colorant- which would have to be the Yttrium. Tom |
|
|
|
Post by tileman2 on Dec 21, 2015 21:45:05 GMT -6
I have long wondered if clay formulas play a role in crystal development. I have been formulating several solely for use with crystalline glaze. In the interim I thought I would put two commercially available porcelains up against each other. Standard cone 10 on the left up against Aardvark on the right. Same firing, same shelf, same glaze mixed then divided for each.  The quarters are for a frame of reference: do you see differences? Edit Note: Fired to cone 6, so not fully mature. Tom |
|
|
|
Post by Koz on Dec 22, 2015 13:47:39 GMT -6
Tom,
I just had to paddle across The River to respond to this one.
Yes, of course clay formulas play a role in crystal development.
Really? You wonder about that? What doesn't?
I hope that helps you eliminate hours upon hours of research and inconsistent results.
And with that being said, we all know that clay and glaze recipes travel about as well as a horse drawn cart with triangular wheels.
Koz
|
|
|
|
Post by tileman2 on Jan 4, 2016 22:31:35 GMT -6
The one thing I do not like about the Midwest: customers always want neutral colors. Offered these people a range, multi colored, gold crystals etc: and they pick brown...  Brown is green at the end of the day. Tom |
|