|
|
Post by tileman2 on Oct 23, 2015 20:18:56 GMT -6
For those wanting to experiment with Moly crystals, I came across an extensive thesis paper from the University of Florida on the topic. It includes formulation, firing, and colorant additions as part of the research. Here is the link: ufdc.ufl.edu/AA00011451/00001/18jLots of good info. Enjoy! Tom |
|
|
|
Post by jfox on Oct 25, 2015 9:09:52 GMT -6
thanks for that article Tom, ive been sitting on a lb of moly for about a year now need to give it shot
|
|
|
|
Post by tileman2 on Oct 26, 2015 16:33:43 GMT -6
Jim:
Was looking for info for that same reason: have a lb of it myself. I have noticed most all on here have some little niche of the crystalline glaze down to a science: yours is silver reduction. That is one mighty fine piece you posted in the main section. Like it so much just might have to buy one of them myself- off the charts excellence- well done sir!!
Tom
|
|
|
|
Post by evan cornish-keefe on Oct 27, 2015 7:52:09 GMT -6
Have you tried these glazes / had much luck with Molybdenum?
I've been messing with MoS2 at cone 6 for a while, but halfheartedly since I had only disappointing results until recently.
I'd like to know more about the effect of the Titanium & Calcium content in the glaze. Also the amount of silica and alumina in recipes I've seen is often in a different ballpark from other macro crystal glazes it seems...?
A number of articles listed in the bibliography look interesting, especially the ones by Myron Altrom and Annelies Kahn, does anyone know where those could be unearthed?
|
|
|
|
Post by tileman2 on Oct 27, 2015 19:49:36 GMT -6
Evan:
I have fired a couple of tests at cone 6 with no success. Been breaking down the chemical composition/s of the of the glaze shown in the article. Actually it has a fair amount of SiO2, if you included the content in Custer and GB. Gerstley also has 20% calcium, which can be added to the whiting addition. So as it stands now: it appears boron and calcium are the two primary fluxes. Then secondary additions of potassium and sodium from the Custer and nitre. The recipe shown is certainly a flux bomb; which I suspect could be narrowed down after some testing. The slow cool from peak down to 1550F also suggest that the perfect Moly hold temp is yet to be discovered. Just a few random thoughts on the fluxing end of this formula.
On the crystal end of it: the zinc has been reduced down into the opacifier range. Moly has a stronger atomic bond than zinc does: which makes it a stronger conductant than zinc. So I view it as dealing with Moly bonding with SiO2 on the same principle as zinc attracting SiO2. Moly has much higher melt temp than zinc: which makes me wonder if the first hold should likewise be higher: if a hold temp would even work. The other interesting point is that Moly is a transition metal, which might affect certain parameters. The higher melt temp is the reason I suspect Boron (borates) are being introduced at higher levels. Think I will replace the nitre with potassium hydroxide; but primarily focus on boron and calcium.
My initial thoughts on putting the pieces of this jigsaw together. A transition metal simply means that how the atoms reform upon cooling is different than regular metals- like zinc. This may play a role, it might not: but it is on my radar. But is also makes me think a higher hold temp is required. All new to me; which makes it even more interesting.
Tom
|
|
|
|
Post by evan cornish-keefe on Nov 9, 2015 13:09:54 GMT -6
Hey Tom, Yeah, looking at the formula in pg 14, glaze CF1, says the boron content is .47 which is a lot. Enough boron to melt at cone 04 : mattanddavesclays.com/Science/UMF%20Boron%20Addition.pdfI think you might be onto something with the hold temp, and I might repeat old tests with that in mind. I thought Zn and Mo are both transition metals, and that Mo has a lower melting point? The closest result i've had so far was a fluke, a piece with a variation of the Fe Ti glaze I mentioned in another thread was next to an unsuccessful Mo test in the kiln, and formed Powellite crystals where it was within 1-2 inches of the Mo test. From there I'm slowly working backwards adding MoS2 and removing Fe and Ti from this glaze. Firing to C5 produces more Mo crystals than at C6. Here's using high-water P5 clay and 0, 5, 10, 15% RIO, with 5%TiO2 and 3% MoS2 FF 3134 - 30.08% Lithium Carb - 16.26% EPK - 21.95% Flint - 31.71   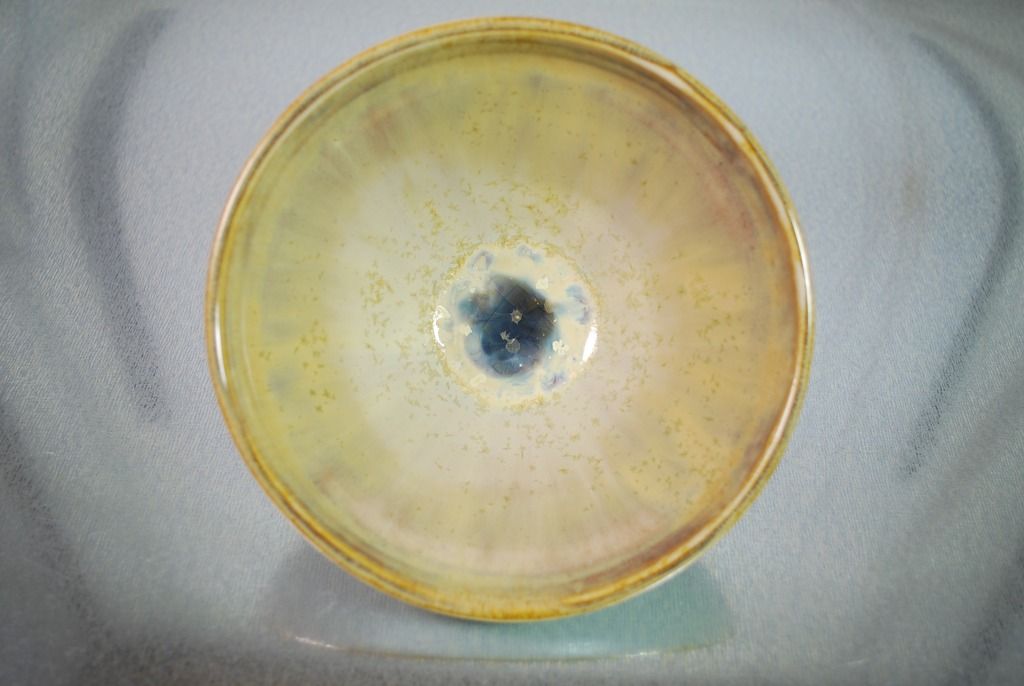     |
|
|
|
Post by tileman2 on Nov 9, 2015 20:55:31 GMT -6
Evan:
I fired a test run a week ago: similar results-without lithium but cone10. Moly B is a sulfide, so I suspect Barium will be a necessary component in this case. After doing some research, strontium might be an alternative in higher % additions. Trying hard to formulate and make substitutions without doing a bunch of tests. In other words, trying to see how much I have learned in the past four years. Again though, Barium I think will be needed to neutralize the effects of sulfides.
Will run a cone 6 at some point shortly; looks like you are dialing in on it at those temps. Not bad at all for the first roll of the dice.
Tom
|
|
|
|
Post by evan cornish-keefe on Nov 11, 2015 10:40:21 GMT -6
Not my first roll of the dice, i've done maybe 15 sets of tests with MoS2, would have done more if my results had given an indication of what direction to continue.
Prior tests all formed crystals, but mostly small rough patches far from the interesting iridescent crystals i was aiming for, I believe those patches are Powellite as well.
What are the effects of sulfides? Barium and Strontium were not materials I was planning to use.
*I accidentally gave you the wrong recipe above, both are variations of another C6 crystal glaze, but the one I tested w/ Mo has less alumina:
Lith Carb - 20
FF 3134 - 37
EPK - 14
Flint - 29
+
TiO2 - 5
MoS2 - 3
RIO - 0, 5, 10, 15
|
|
|
|
Post by frederi on Nov 11, 2015 12:13:28 GMT -6
Evan,
If you don't want the sulfur you could roast the MoS2 in air for about 10 minutes at 760Deg C. getting MoO3. This sublimes so higher temperatures will cause more loss. The secret ingredient for developing a moly glaze is persistence. Dead ends abound.
Gordon
|
|
|
|
Post by tileman2 on Nov 11, 2015 17:40:33 GMT -6
Evan:
Zinc is a chalcophile element that combines readily with sulfur- which is why the presence of trace levels of sulfur do not react with zinc silicate glazes. Molybdenum is a siderophile element which reacts strongly to sulfur. The problem then becomes that the sulfur reacts with other ingredients so the effects of that sulfur has to neutralized; and barium is the only flux that will do that. Strontium is the closest relative to barium; the only reason I suggested it. My thought is the barium does in this glaze combination what antimony oxide does in glass. My thoughts are worth two cents, and for today only I am having a half price sale.
If the topic interests you: Goldschmidt classification of minerals.
Tom
|
|
|
|
Post by jfox on Nov 12, 2015 20:43:50 GMT -6
|
|
|
|
Post by tileman2 on Nov 16, 2015 20:01:10 GMT -6
Evan: This is a cone 6 attempt at moly crystals. The recipe shown in the article was used: the nitre was removed- then 7% lithium and 5% strontium added. You can see the beginnings of moly crystals in a few places. The sulfur causes crawling; which is what is used to create crawl glazes. While the strontium did help; still think barium will do the job much better. This represents my 2nd attempt at cone 6 moly- the first one was just a boiled mess. Headed in the right direction, but not there yet.  Tom |
|
|
|
Post by tileman2 on Nov 25, 2015 17:45:33 GMT -6
Evan: Here is my third attempt at Cone 6 Moly crystals. Threw this piece in with a regular cone 6 firing load, so obviously temps need to be adjusted. Yes, you need some barium additions.  Still needs some tweaking, adjustments in ramp temps: but not bad at all for attempt #3. Tom |
|
|
|
Post by jfox on Nov 25, 2015 18:56:53 GMT -6
|
|
|
|
Post by jfox on Nov 27, 2015 0:58:39 GMT -6
|
|